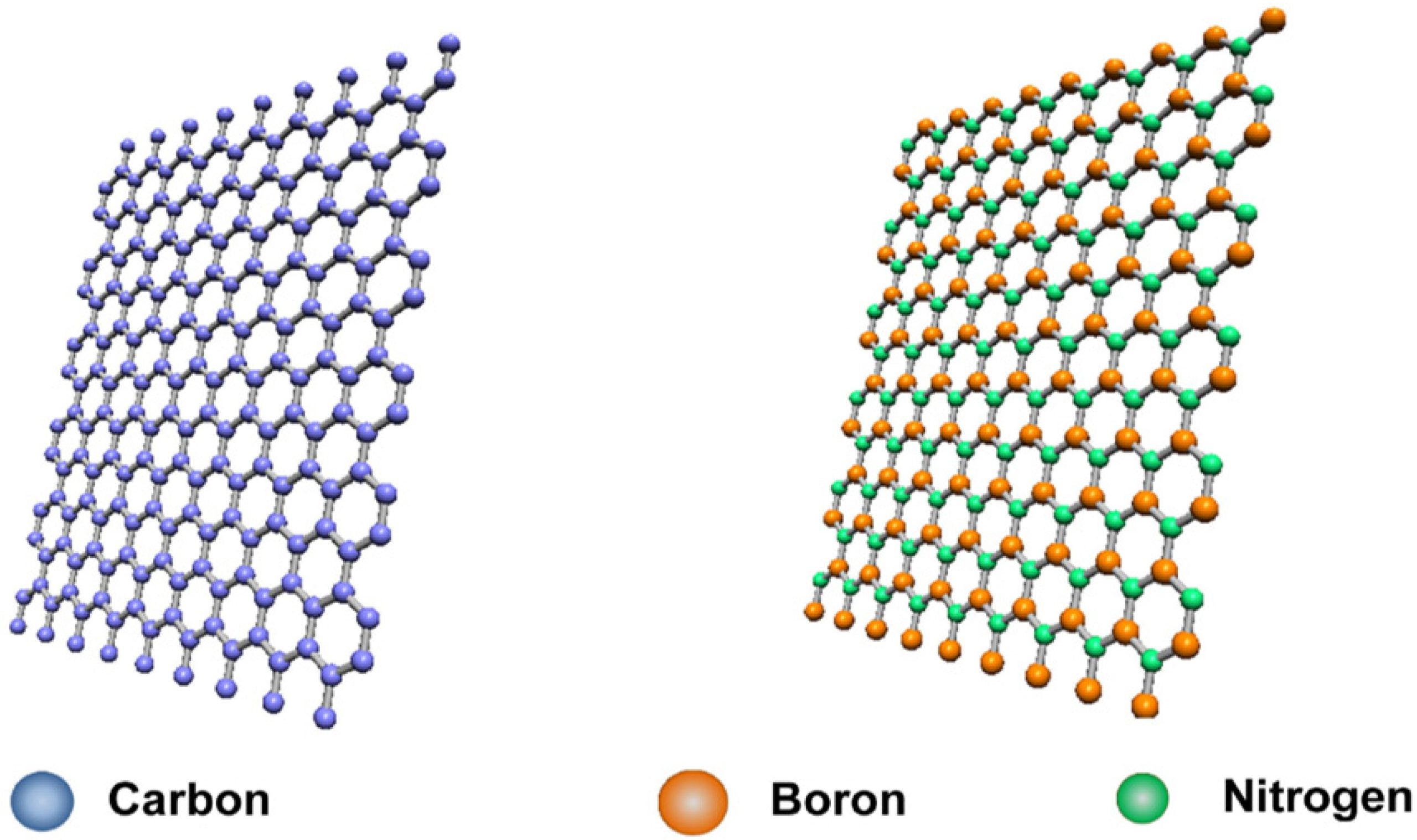
Boron Nitride is a binary compound composed of boron (B) and nitrogen (N) with a 1:1 atomic ratio. It is often compared to carbon because it forms several analogous structures (like graphite and diamond), each with distinct properties.
Materials of Boron Nitride
Typical raw materials used in the synthesis of Boron Nitride include:
1. Boron-containing precursors
- Boric acid (H₃BO₃)
- Boron oxide (B₂O₃)
- Boron trioxide
- Elemental boron powder
- Boron halides (e.g., BCl₃, BBr₃) for CVD processes
2. Nitrogen sources
- Ammonia gas (NH₃)
- Nitrogen gas (N₂)
- Ammonium salts (e.g., NH₄Cl, NH₄HCO₃)
- Urea (CO(NH₂)₂)
- Hydrazine derivatives in specialized syntheses

3. Catalysts and Additives (when applicable)
- Alkali metal catalysts for low-temperature formation
- Growth promoters for c-BN (e.g., Li₃N, Ca₃N₂)
- Carbon-based templates for Boron Nitride nanotubes/nanosheets
4. Substrate Materials (for thin films)
- Silicon wafers (Si, SiO₂/Si)
- Sapphire (Al₂O₃)
- Copper and nickel foils
- Transition-metal substrates for CVD growth
5. High-pressure/high-temperature (HPHT) media
- Graphite heaters
- Refractory metal capsules (e.g., W, Mo)
- Cubic or belt-type pressure anvils for c-BN synthesis
Methods of Boron Nitride
1. Chemical Vapor Deposition (CVD)
Boron Nitride is favorite high-tech manufacturing route.
- Process: A boron-containing vapor (e.g., BCl3 or BF3) reacts with NH3 or N2 at high temperature.
- Outcome: Hexagonal Boron Nitride films, nanosheets, coatings.
- Uses: Electronics, dielectric layers, 2D materials.
2. Carbothermal Nitridation
A fancy way of saying “heat boron oxide with carbon and nitrogen until it stops looking like either one.”
- Reactants: B2O3 + carbon + N2/NH3
- Temperature: 1400 to 1800°C
- Product: Mostly hexagonal Boron Nitride powders.
3. Polymer-Derived Ceramics (PDC) Route
Mix chemicals until they form a polymer, then torch the polymer until it becomes ceramic.
Steps:
- Start with borazine or melamine-based polymers
- Crosslink the polymer
- Pyrolyze under nitrogen at 900 to 1500°C
Good for: Boron Nitride fibers, porous Boron Nitride, and shapes with better control.
4. High-Pressure High-Temperature (HPHT) Method
This is how you bully Boron Nitride into becoming cubic Boron Nitride (its diamond-wannabe form).
- Conditions: Above 5 GPa and 1500+°C
- Outcome: Cubic Boron Nitride (c-BN), extremely hard, used for cutting tools.
5. Mechanical Methods
For those who don’t mind a bit of noise.
- Ball milling: Reduces particle size, introduces defects
- Hot pressing / spark plasma sintering: Densifies Boron Nitride powders into ceramics
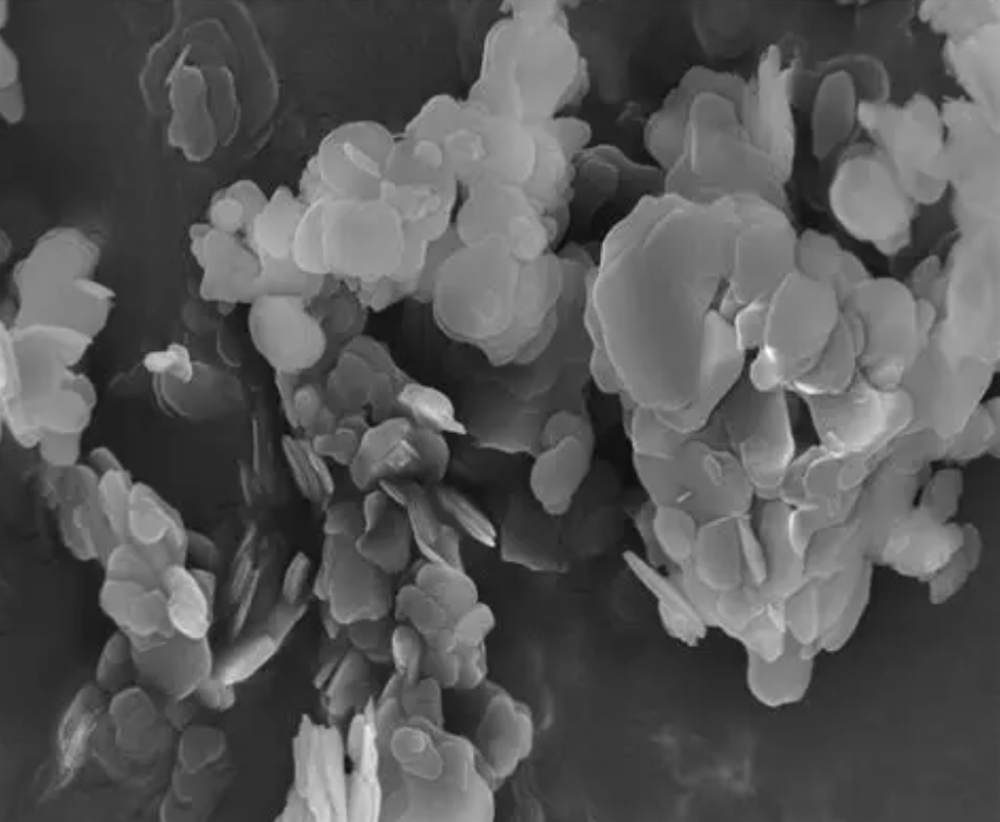
6. Characterization Methods
Because scientists don’t believe anything unless you throw instruments at it:
- XRD: Check crystal phases
- SEM/TEM: Observe morphology
- FTIR/Raman: Confirm chemical bonding
- BET: Surface area
- TGA: Thermal stability
Summary
This overview provides a full Materials and Methods framework for Boron Nitride, covering precursor chemicals, synthesis routes for different allotropes, film deposition techniques, nanostructure fabrication, purification, and standard characterization workflows.
