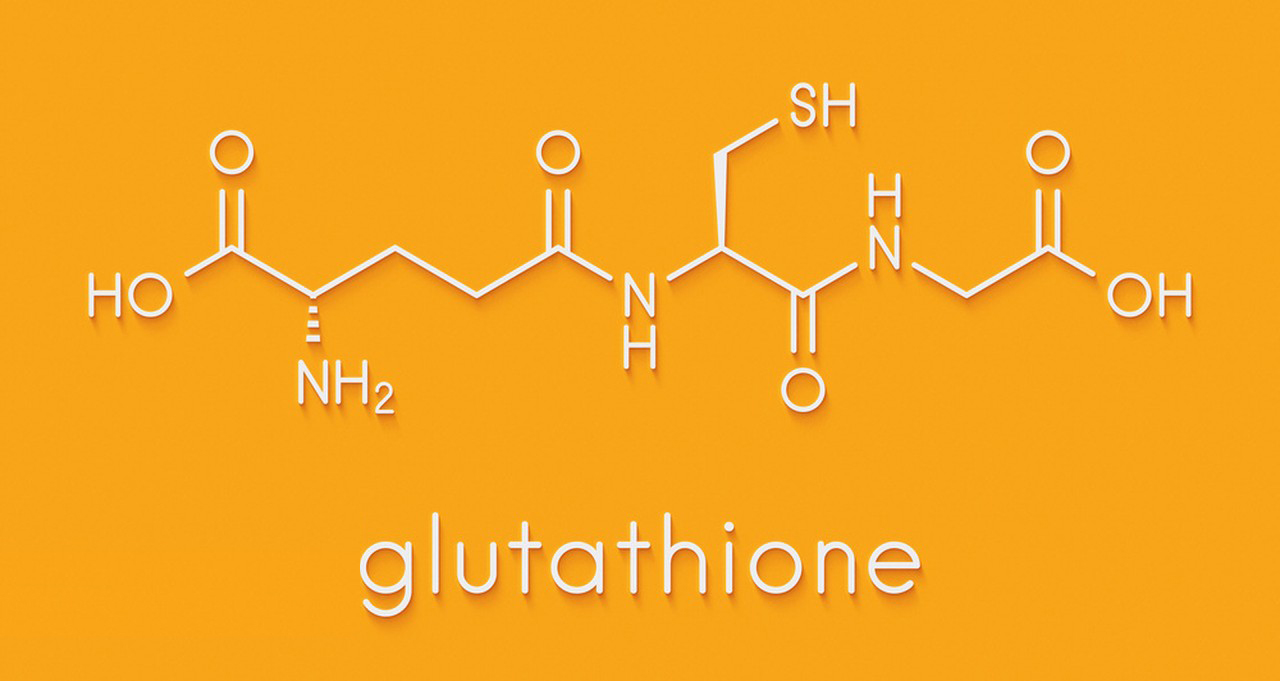
Glutathione is a tripeptide composed of three amino acids: cysteine, glutamic acid, and glycine. Its chemical structure is represented as follows:
H2N-(Cys-Gly-Glu)-COOH
Here, Cys represents cysteine, Gly represents glycine, and Glu represents glutamic acid. Glutathione is often abbreviated as GSH.
Some important physical properties of glutathione include:
Molecular Weight: The molecular weight of glutathione is approximately 307.32 g/mol.
Solubility: Glutathione is soluble in water and polar solvents due to its hydrophilic nature.
Melting Point: The melting point of glutathione can vary depending on the form (reduced or oxidized) and the hydration state. For reduced glutathione (GSH), the melting point is approximately 185-195°C.
Color: Reduced glutathione is typically colorless, while the oxidized form (glutathione disulfide, GSSG) can appear yellow due to the presence of disulfide bonds.
pKa Values: Glutathione contains ionizable functional groups (amino and carboxyl groups). The pKa values of its ionizable groups are around 2.1 (carboxyl group of glutamic acid), 2.2 (amino group of cysteine), and 8.7 (amino group of glycine).
Redox Potential: Glutathione plays a crucial role in cellular redox processes. The redox potential of the GSH/GSSG couple is around -240 mV, making it an important antioxidant in biological systems.
Biological Functions: Glutathione is a vital molecule involved in various cellular processes, including antioxidant defense, detoxification, and regulation of cell signaling. It helps protect cells from oxidative stress by scavenging free radicals and participating in enzymatic reactions that neutralize reactive oxygen species.
Thiol Group: The cysteine residue in glutathione contains a thiol (-SH) group, which is highly reactive and can readily form disulfide bonds with other thiols. This thiol group is essential for the molecule’s antioxidant properties.
Intracellular Concentration: Glutathione is present in cells at relatively high concentrations compared to other antioxidants. Its levels can fluctuate based on various factors such as age, diet, and exposure to oxidative stressors.
It’s important to note that glutathione exists in both reduced (GSH) and oxidized (GSSG) forms within cells, and the balance between these forms is crucial for its function. GSH acts as a reducing agent, while GSSG represents the oxidized, dimeric form that is often used to regenerate GSH through enzymatic reactions.
