Hydroxyapatite (HA) is a calcium phosphate mineral that makes up a significant portion of the human bone and teeth. Here are some key details about the chemical structure and physical properties of hydroxyapatite:
Chemical Structure of Hydroxyapatite:
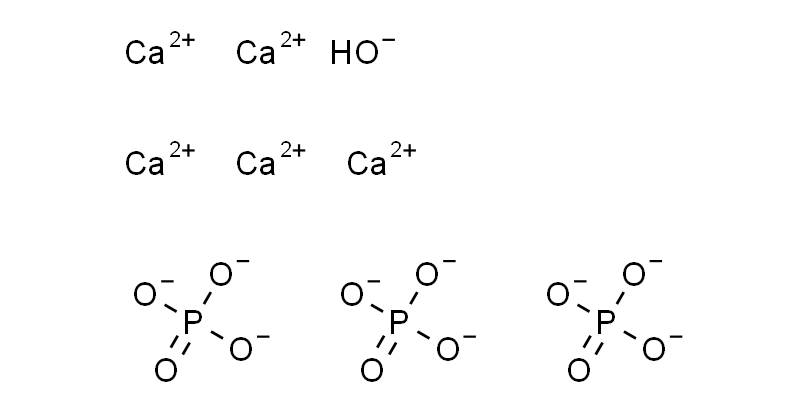
Hydroxyapatite has a chemical formula represented as Ca₅(PO₄)₃(OH), indicating its composition of calcium, phosphorus, and hydroxyl ions.
The crystal structure of hydroxyapatite is hexagonal.
Chemical Composition:
Calcium (Ca): 39.86%
Phosphorus (P): 18.43%
Oxygen (O): 40.71%
Hydrogen (H): 1.00%
Physical Properties of Hydroxyapatite:
1.Color: Hydroxyapatite is usually white or colorless.
2.Hardness: It is a hard mineral with a Mohs hardness of 5.
3.Density: The density of hydroxyapatite varies, but it is generally around 3.16 g/cm³.
4.Melting Point: Hydroxyapatite decomposes before melting. It starts to decompose around 1,100°C.
5.Solubility: Hydroxyapatite is sparingly soluble in water and acidic solutions. It dissolves more readily in acidic conditions.
Biological Significance of Hydroxyapatite:
Hydroxyapatite is a crucial component of bones and teeth, providing strength and rigidity to these tissues.
It plays a role in the mineralization of bones and teeth during the formation and growth of these structures.
In biomedical applications, hydroxyapatite is used in bone grafts, dental implants, and other medical implants due to its biocompatibility.

Applications of Hydroxyapatite:
Biomedical implants and scaffolds for bone tissue engineering.
Dental materials, such as in the fabrication of dental implants and coatings for dental prosthetics.
Biomimetic materials for bone repair and regeneration.
Understanding the chemical structure and physical properties of hydroxyapatite is essential for its various applications, particularly in the field of biomaterials and medicine.
