As of my last knowledge update in January 2022, semaglutide is a medication used in the treatment of type 2 diabetes. It is a glucagon-like peptide-1 (GLP-1) receptor agonist, and its molecular structure is similar to the natural hormone GLP-1 that is released in response to food intake.
Please note that there might be updates or new information beyond my last training data, so it’s essential to refer to the latest literature, drug labels, or consult a healthcare professional for the most recent and accurate information.
Here is a general overview of the materials and methods related to semaglutide:
Materials of Semaglutide:
1.Active Ingredient:
Semaglutide is the active pharmaceutical ingredient (API) in the medication.
2.Excipients:
Excipients are inactive components added to the formulation to enhance stability, absorption, or other characteristics of the drug. Common excipients in pharmaceutical formulations may include bulking agents, stabilizers, preservatives, etc.
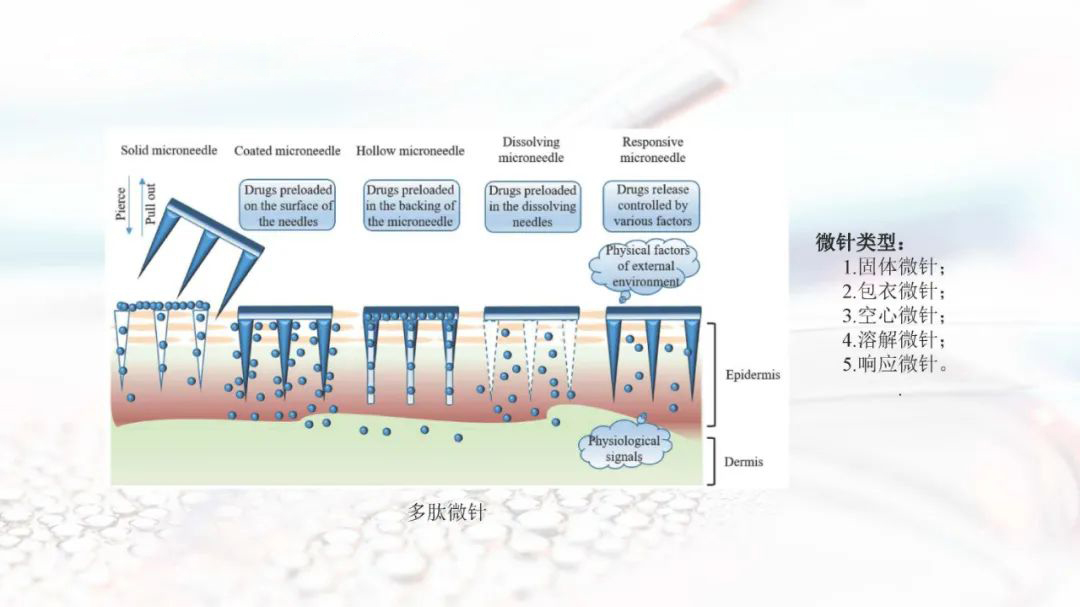
Methods of Semaglutide:
1.Synthesis:
The chemical synthesis of semaglutide involves the production of the peptide chain with the correct amino acid sequence. This process typically includes solid-phase peptide synthesis (SPPS) or other suitable methods.
2.Formulation:
The active ingredient (semaglutide) is formulated into a dosage form suitable for administration. This can include tablet formulations, injectable solutions, or other appropriate forms based on the route of administration.
3.Analytical Methods:
Rigorous analytical methods are employed to ensure the quality and purity of semaglutide. High-performance liquid chromatography (HPLC), mass spectrometry, and other analytical techniques may be used to assess the identity and purity of the drug.
4.Clinical Trials:
Semaglutide, like any new medication, undergoes extensive clinical trials to evaluate its safety and efficacy. These trials involve testing the drug on human subjects under controlled conditions.
5.Dosage and Administration:
The recommended dosage and administration guidelines for semaglutide are established based on the results of clinical trials. This includes information on the frequency of administration, dosage strength, and any specific instructions for use.
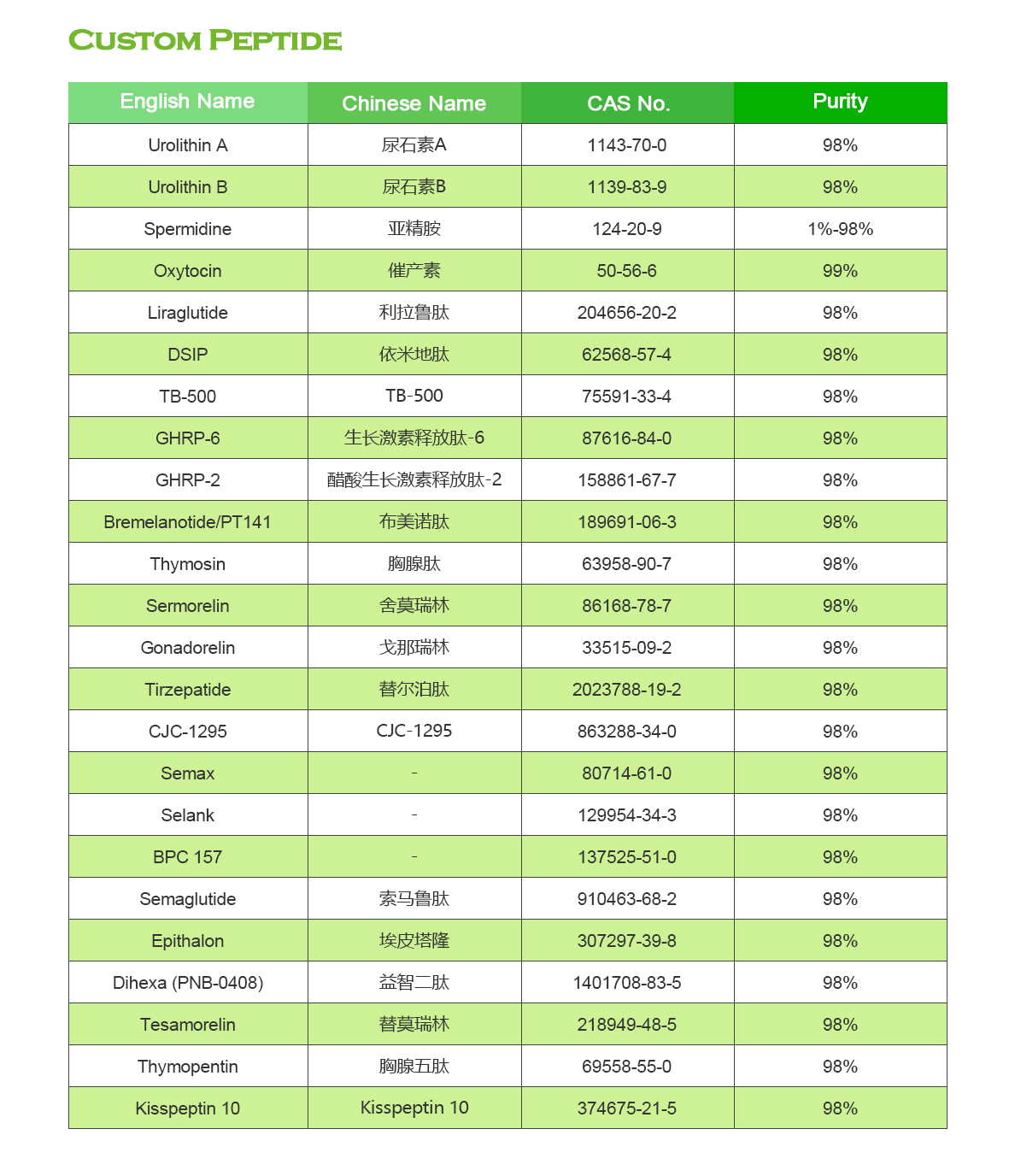
6.Regulatory Approval:
The drug undergoes regulatory review and approval by health authorities such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). This involves submission of detailed data on the safety, efficacy, and quality of the drug.
Always consult the most recent and specific sources, such as the drug label or prescribing information, for the latest details on semaglutide or any medication.
