Origin of Benfotiamine
Benfotiamine is a synthetic S-acyl derivative of thiamine (vitamin B1).
It was developed in Japan in the 1960s–1970s as part of a research trend to create lipid-soluble thiamine analogs that could bypass poor intestinal absorption of regular thiamine.
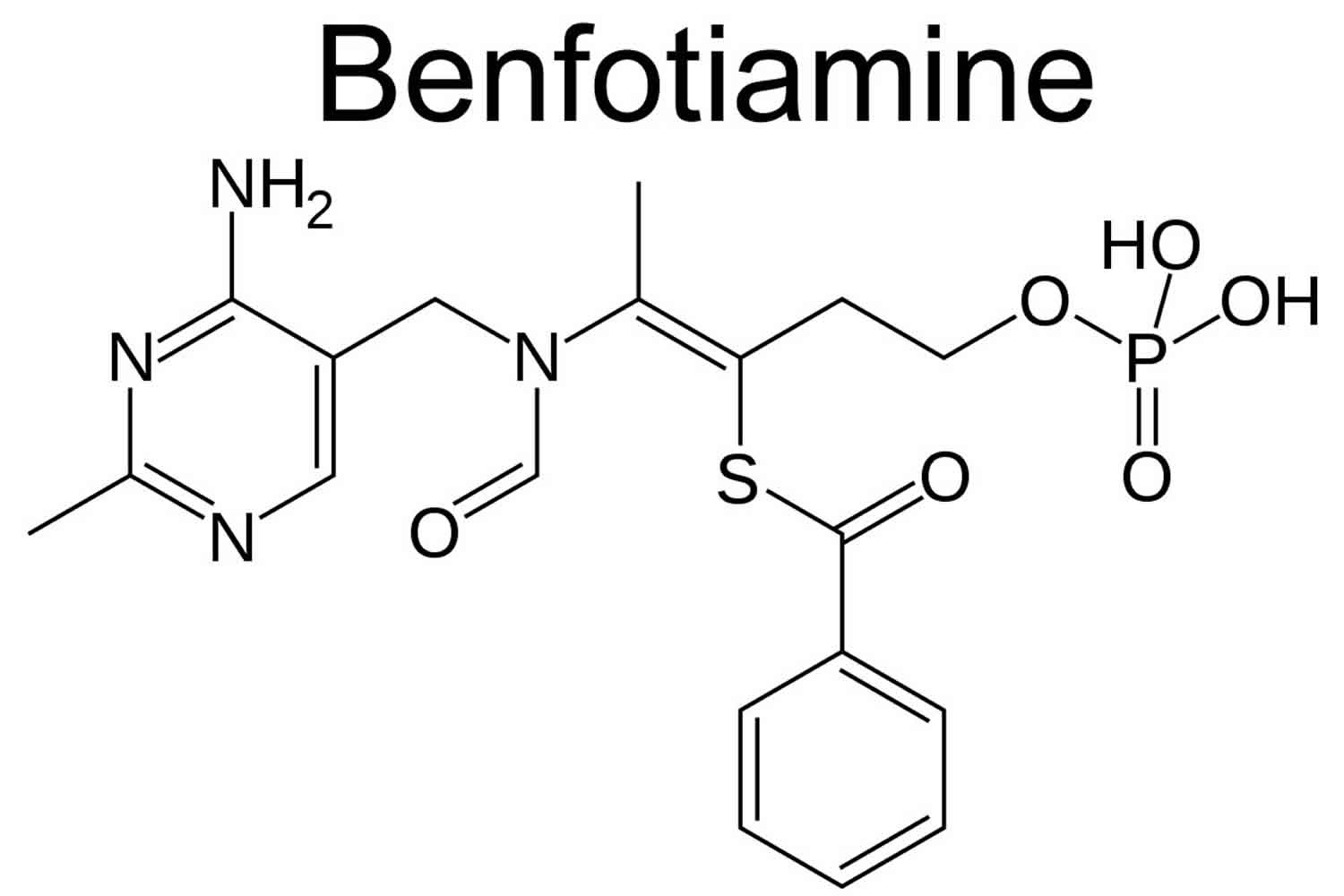
Chemically it is:
S-benzoylthiamine O-monophosphate
Benfotiamine ultimately is converted in the body → to thiamine (bent into TPP) which is the active coenzyme form.
Introduction / Purpose of Benfotiamine
Ordinary thiamine (B1) is water soluble → poor penetration into cells and limited plasma half life.
Benfotiamine was designed specifically to:
- Increase bioavailability
- Cross cell membranes more efficiently
- Provide higher intracellular thiamine diphosphate (TDP = active cofactor form of B1) concentrations
When ingested, benfotiamine is dephosphorylated, absorbed via the intestinal tract, converted to S-benzoylthiamine → then to thiamine → then phosphorylated inside cells to TDP.
Benfotiamine is NOT used to correct “plain” B1 deficiency per se.
It is used when intracellular metabolic activation is targeted (especially in hyperglycemia-related metabolic stress).
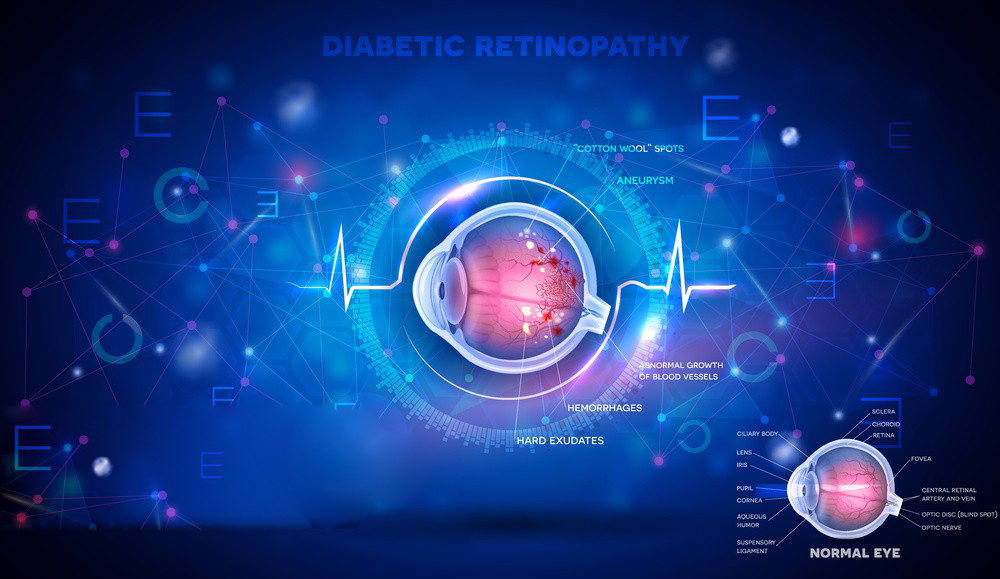
The most studied medical usage is in diabetic neuropathy.
Properties of Benfotiamine
| Property | Description |
| Solubility | Lipid-soluble (unlike thiamine chloride) |
| Absorption | High oral absorption; active transport + passive penetration |
| Bioavailability | Can produce 5–20× higher blood thiamine availability vs. thiamine salts |
| Active form produced in body | Thiamine diphosphate (TDP) |
| Mechanistic signature | Inhibits formation of advanced glycation end products (AGEs) via hexosamine / pentose phosphate / transketolase pathway activation |
| Stability | Chemically more stable than thiamine chloride in gastric acid |
Why benfotiamine is special (summary sentence)
Benfotiamine is basically the “engineered, lipid-soluble, pharmacological B1” created to push thiamine into intracellular metabolic pathways at a deeper level than conventional B1.
If you want, I can now continue one of these directions:
- detailed pharmacokinetics (ADME)
- exact chemical structure description and key functional groups
- clinical applications overview
D) compare benfotiamine vs. allithiamine/tetrahydrofurfuryl thiamine (TTFD) (this is a GOOD comparison, because people confuse these two)
Which direction do you want next?
