Avobenzone is a widely used ingredient in cosmetics, particularly in sunscreens, due to its ability to absorb and filter UVA (ultraviolet A) rays. Here’s an overview of its role in cosmetics:
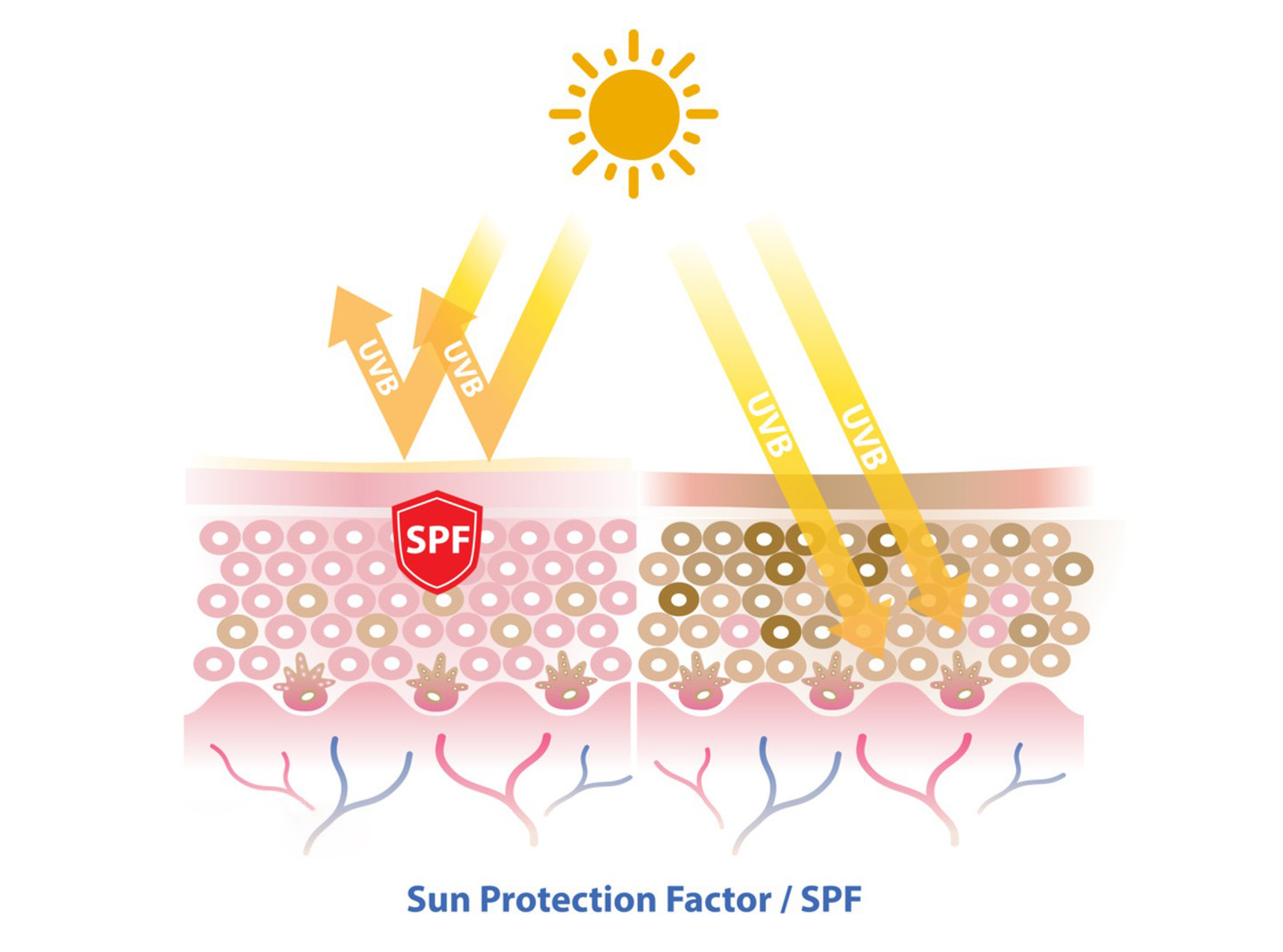
- UVA Protection: Avobenzone is an effective broad-spectrum sunscreen agent that primarily protects the skin from UVA radiation. UVA rays penetrate the skin deeply and contribute to premature skin aging, wrinkles, and skin cancers. By absorbing these harmful rays, avobenzone helps prevent sunburn and other sun-related skin damage.
- Photostability: Avobenzone is known for its photostability, meaning it remains effective even after being exposed to sunlight for prolonged periods. However, it can break down when exposed to UV light over time, which is why it’s often combined with other stabilizing agents to improve its effectiveness.
- Anti-aging Properties: By preventing UV-induced skin damage, avobenzone helps reduce the visible signs of aging, such as fine lines, wrinkles, and age spots. This makes it a common ingredient in anti-aging skincare products.
- Compatibility: Avobenzone is compatible with other sunscreen agents, often combined with ingredients like octinoxate, octocrylene, and homosalate to provide broad-spectrum UV protection, ensuring coverage against both UVA and UVB rays.
- Cosmetic Formulation: In addition to sunscreens, avobenzone is often included in other cosmetic products, such as foundations, moisturizers, and lip balms, to offer UV protection.
Overall, its primary role is to protect the skin from UVA radiation, making it an essential component in many sun protection and anti-aging cosmetic formulations.
Synthesis of Avobenzone
Avobenzone is a commonly used organic compound in sunscreens and cosmetic products due to its ability to absorb UV radiation, particularly UVA rays. The synthesis of Avobenzone (also known as Butyl Methoxydibenzoylmethane) involves a multi-step organic reaction.
Here is a basic outline of how Avobenzone can be synthesized:
Materials:
- 4-hydroxybenzophenone (Benzophenone-3)
- butyl-4-hydroxybenzoic acid
- Reagents: Acid chlorides, bases, and solvents like dichloromethane (DCM), and DMF.
General Synthetic Scheme:
1.Preparation of Intermediate:
- A typical first step involves preparing a benzophenone intermediate. For instance, benzophenone is reacted with 2-butyl-4-hydroxybenzoic acid (usually with a dehydrating agent like thionyl chloride or oxalyl chloride) to form a butylated benzophenone derivative.
2.Aldol Condensation (if necessary):
- If a more complex substitution is required to introduce a functional group in the structure, an aldol condensation may occur. However, in this case, this step may be skipped in favor of direct acylation.
3.Acylation of the Hydroxyl Group:
- The hydroxyl group on the aromatic ring reacts with an acylating agent (such as acyl chloride, typically a methoxycarbonyl group) to form a substituted dibenzoylmethane structure.
4.Purification:
- The final product, Avobenzone, is purified using methods such as recrystallization or chromatography.

Key Reactions:
- Esterification or acylation reaction is critical for attaching the butyl and methoxy groups to the aromatic rings.
- Solvent choice (such as DCM or acetone) plays an important role in ensuring the reaction goes to completion while keeping side reactions minimal.
The synthesis of Avobenzone is a well-established procedure in the field of organic chemistry, typically involving controlled reactions under mild conditions.
