Boron Nitride (BN) exists in several polymorphs (most commonly Hexagonal Boron Nitride — h-BN, plus Cubic Boron Nitride — c-BN, Wurtzite Boron Nitride — w-BN, and various amorphous/nanostructured forms). Below I’ll summarize the major synthesis routes, typical conditions (ranges), advantages/limitations, and common characterization methods so you can pick the right route for your target Boron Nitride type and scale.
1) Routes by product type
Hexagonal Boron Nitride (h-BN) — bulk / powders / films / nanosheets
1. Direct nitridation (gas-solid)
- Method: nitridation of boron/boron oxide using ammonia (NH₃) or nitrogen sources at high temperature.
- Typical conditions: 900–1600 °C in flowing NH₃ or N₂/NH₃ mixtures; reaction time and ramp depend on particle size.
- Pros: simple, scalable for powders; inexpensive precursors.
- Cons: may produce oxygen-containing impurities if B₂O₃ is present; requires high T for good crystallinity.
2. Polymer-derived ceramics (PDC) / precursor pyrolysis
- Method: synthesize boron-nitrogen polymer (e.g., polyborazylene, polyborazine), then pyrolyze under inert/amine atmosphere to form Boron Nitride.
- Typical conditions: pyrolysis 800–1200 °C; allows shaping before conversion.
- Pros: good control of composition, useful for fibers/coatings and shaped parts.
- Cons: precursor synthesis required; organic byproducts must be handled.

3. Chemical vapor deposition (CVD) — films and 2D h-Boron Nitride
- Method: decompose boron-containing vapors (borazine, BCl₃ + NH₃, diborane + NH₃, etc.) onto heated substrates.
- Typical conditions: substrate temps commonly 800–1200 °C (depends on precursor). Low-pressure or atmospheric CVD variants exist.
- Pros: high-quality, uniform films and monolayer/bilayer control for electronics.
- Cons: needs careful precursor handling and substrate choice; process optimization required.
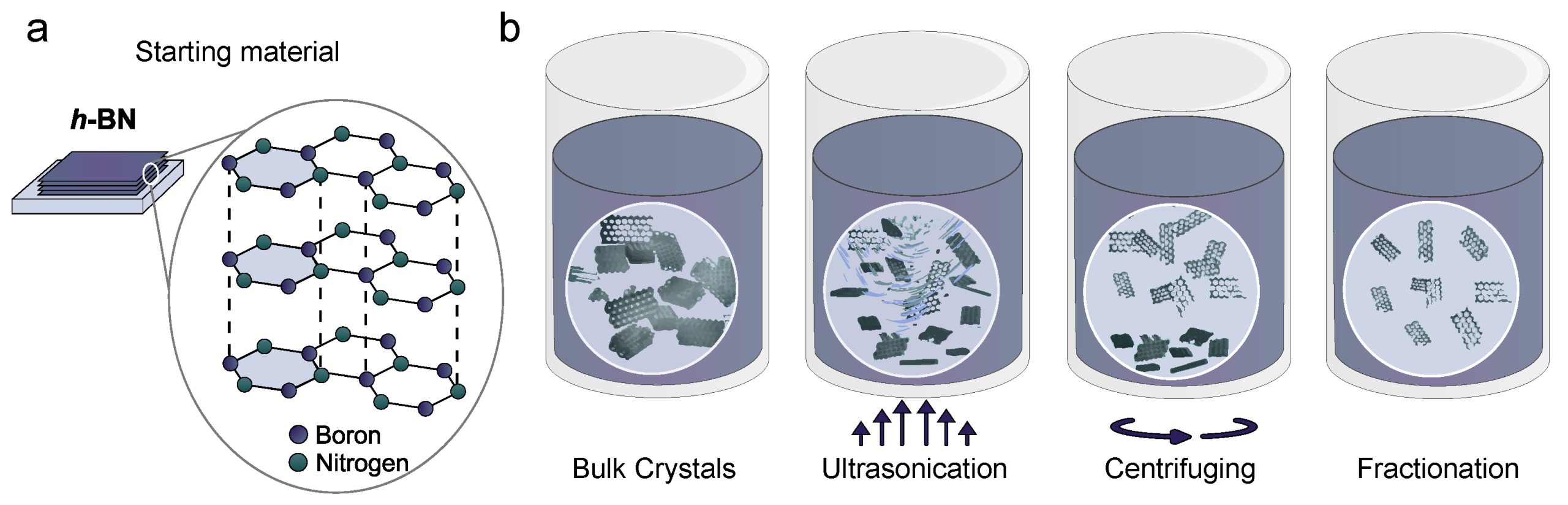
4. Liquid/solvothermal and precursor routes for nanosheets
- Method: solvothermal synthesis, exfoliation of bulk h-BN, or bottom-up reactions using precursors in autoclaves at modest temperatures (≤400 °C).
- Pros: lower temperature, can give nanosheets or nanoparticles.
- Cons: may require post-treatment to remove residues and improve crystallinity.
5. Ball-milling + annealing
- Method: mechanochemical mixing of boron/boron oxide with nitrogen source followed by thermal annealing.
- Pros: inexpensive, scalable for powders.
- Cons: defects and contamination from milling media; needs high-T anneal for crystallinity.
Cubic Boron Nitride (c-BN) and wurtzite Boron Nitride (w-BN) — superhard phases
High-pressure, high-temperature (HPHT) synthesis
- Method: convert h-BN or B + N precursors to sp³-bonded phases under extreme pressures and temperatures, often with catalysts.
- Typical conditions: very high pressures and temperatures (HPHT laboratory conditions).
- Pros: yields superhard c-Boron Nitride used industrially for cutting/grinding.
- Cons: requires specialized HPHT equipment and catalysts; not accessible in standard labs.
2) Typical precursors and reagents
- Boron sources: elemental boron, boron oxide (B₂O₃), boron halides (BCl₃), diborane (B₂H₆), borazine or polyborazylene (B₃N₃H₆ and derivatives).
- Nitrogen sources: ammonia (NH₃), molecular N₂ (less reactive), borazine (both B and N), urea/amine precursors (in solvothermal).
- Catalysts / additives (process dependent): metal substrates or seeds in CVD; for HPHT conversion metallic catalysts are used (industrial c-BN routes).
3) Typical processing parameters (guideline ranges)
- Temperature: 400 °C (solvothermal/exfoliation) → 800–1600 °C (pyrolysis/nitridation/CVD) → much higher + high pressure for c-BN/w-BN.
- Atmosphere: inert (Ar, N₂) or reactive (NH₃, NH₃/H₂ mixtures); oxygen must be excluded for high-purity Boron Nitride.
- Pressure: near atmospheric for most CVD and nitridation; elevated pressure only for HPHT c-Boron Nitride synthesis.
- Time: minutes–hours (CVD film growth); hours for bulk nitridation/pyrolysis; depends on thickness/crystallinity target.
4) Key practical considerations
- Purity control: remove boron oxide or oxygen contamination by using dry precursors and inert atmospheres; post-annealing in NH₃ can reduce oxygen.
- Crystallinity vs. defects: higher temperature and longer anneal improve crystallinity but can increase grain growth (affects nanosheet yield).
- Substrate choice (CVD): metals (Cu, Ni) or transition metal foils are common for 2D h-BN growth; insulating substrates require a transfer step.
- Scale: powder production (nitridation, PDC routes) scales well; large-area monolayer films require optimized CVD reactors.
5) Characterization methods
- X-ray diffraction (XRD): phase identification (h-BN peaks vs c-BN).
- Raman spectroscopy: h-BN E₂g mode (~1366 cm⁻¹ for high-quality h-BN).
- TEM / SEM: morphology, layer count, crystal defects.
- XPS / FTIR: chemical bonding, impurities (oxygen, carbon).
- AFM: thickness of 2D flakes/films.
6) Common applications tied to synthesis choice
- High-quality 2D h-BN (electronic/insulator layers): low-pressure CVD with borazine or BCl₃/NH₃ on metal substrates.
- Bulk powders, lubricants, cosmetics: large-scale nitridation or PDC routes.
- Superhard tooling (c-Boron Nitride): HPHT synthesis in specialized facilities.
- Ceramic composites & coatings: polymer-derived Boron Nitride coatings and powder additions.
7) Safety & environmental notes
Handle NH₃, BCl₃, diborane, and boron halides with appropriate gas-handling systems and scrubbing. These are toxic/corrosive and in some cases pyrophoric (diborane). Use proper ventilation, gas monitors, and PPE.
- Waste: acidic/corrosive byproducts (from halides) must be neutralized and disposed of per regulations.
