(UDCA) as a weapon in the fight against COVID-19. UDCA was originally approved by the FDA in the late 1980s as a treatment for liver disease, and it is now available as a generic drug. However, recent research has suggested that UDCA may have broader applications in the fight against infectious diseases. In a study published in the prestigious journal “Nature” in December 2022, researchers from the University of Cambridge found that UDCA could inhibit the ACE2 receptor, a key protein that allows the SARS-CoV-2 virus to enter human cells. By blocking this receptor, UDCA could potentially prevent or limit the severity of COVID-19 infections. What’s more, the researchers believe that UDCA could also be effective against future mutations of the virus and other coronaviruses. While most UDCA on the market is currently synthesized chemically, there is growing What is the current availability of UDCA as a treatment for COVID-19? Are there any potential side effects or risks associated with using UDCA to combat COVID-19?interested in using a biocatalytic approach to produce UDCA from chenodeoxycholic acid (CDCA), a related compound? This method has several advantages, including lower cost and a shorter synthesis pathway. By using enzymes to convert CDCA to UDCA, it is possible to produce the drug on a larger scale and at a lower cost. As the world continues to grapple with the ongoing COVID-19 pandemic, UDCA could become an important tool in our fight against the virus. Whether you are a scientist, a healthcare professional, or simply someone interested in staying up-to-date on the latest developments in infectious disease research, this is a topic worth exploring in more detail. With its potential to inhibit viral entry and prevent or limit the severity of COVID-19 infections, UDCA could be a game-changer in the fight against this devastating disease.
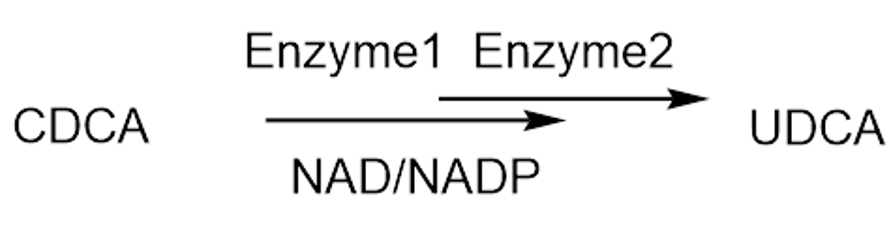
(Biocatalytic Synthesis of UDCA)
